Abstract
OBJECTIVES: While chemotherapy is fundamental to the treatment of many cancers, it is associated with adverse events (AEs), including anemia. CIA is linked to many symptoms, including fatigue and cognitive impairment. Most cancer patients receiving chemotherapy experience CIA. ESAs are indicated for treatment of CIA in non-myeloid cancers. Given the extensive data available, we reviewed SRs of the existing evidence on ESAs for treatment of CIA, to assess their relative efficacy and safety.
METHODS: A search for published SRs of ESAs for the treatment of CIA in adults with non-myeloid cancers was conducted across seven databases and three online resources, from inception to July 2020. Data were extracted on hemoglobin-related (Hb)-outcomes; mortality; health-related quality of life (HRQoL), iron use, red blood cell (RBC) transfusions, cancer-related outcomes, and serious AEs (SAEs) including thromboembolic events. Risk of bias was assessed by two reviewers using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) 2 tool.
RESULTS: Of 1027 records assessed, 33 records met eligibility criteria; from these, 14 SRs (from 19 publications) were considered core and were extracted. The majority of SRs (n=13) were meta-analyses of randomized, controlled trials. Mortality was evaluated in 9 SRs: 5 reported no difference between ESA vs. control (best supportive care (BSC) and/or placebo and/or no treatment) and 2 reported higher mortality with ESA vs. control (standard care (no iron or oral iron) and/or placebo and/or no treatment). Cancer-related overall survival was not significantly different for ESA vs. control (BSC or placebo) in 2 SRs, or vs. no ESA treatment in 1 SR. Relapse-free, recurrence-free and progression-free survival did not differ between ESA vs. BSC, no treatment, or placebo, according to 1 SR.
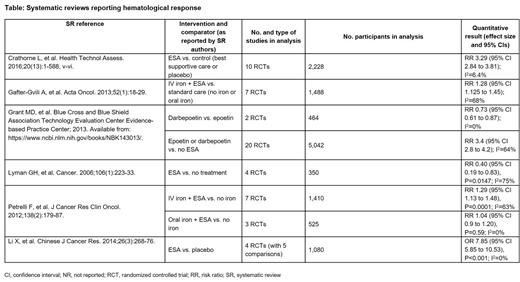
Two SRs reported a significantly improved change in Hb from baseline for ESA vs. placebo or BSC. Four SRs reported significantly improved hematological response (definitions varied by study) for ESA vs. control (BSC and/or placebo and/or no treatment) (Table). RBC transfusion requirements were reported for 5 treatment comparisons in 12 SRs. For 3 comparisons (ESA vs. control (BSC and/or placebo and/or no treatment); IV iron + ESA vs. standard care; and IV iron + ESA vs. no iron + ESA), there was a consistent statistically significant reduction in transfusion rates with ESA-based treatment. For 2 other comparisons, 1 SR reported no significant difference in transfusion rates with oral iron + ESA vs. no iron + ESA, and 2 SRs found no difference between darbepoetin vs. epoetin. ESA was associated with a requirement for significantly fewer RBC units per patient vs. control (BSC or placebo) in 1 SR.
Functional Assessment of Cancer Therapy-Anemia (FACT-An, 6 SRs) and FACT-Fatigue (FACT-F, 7 SRs) were the only HRQoL measures reported. Two SRs reported clinically important improvements in FACT-An with ESA vs. control (BSC and/or placebo); the remaining SRs reported difficulty in interpreting results due to study heterogeneity. Five SRs reported significant improvements in FACT-F with ESA vs. control (BSC and/or placebo and/or no treatment). One SR did not present a meta-analysis, stating that FACT-F results did not differ for ESA vs. placebo groups, and 1 SR reported a significant improvement in FACT-F for IV iron + ESA vs. standard care (no iron or oral iron).
Of 2 SRs reporting SAEs, 1 found no difference in incidence for IV iron + ESA vs. standard care (no iron or oral iron) and 1 found significantly greater incidence for ESA vs. no treatment. Of 6 SRs that compared ESA vs. control, 3 reported an increased risk of thromboembolic events with ESAs in the treatment of CIA and 2 reported no significant difference between treatment groups. The remaining SR reported numerically higher incidences of thromboembolic events with ESA vs. control (no statistical comparison).
Several outcomes of interest were not reported, including time to first rescue therapy and supplementary iron use. Most SRs did not report on study quality.
CONCLUSIONS: In all SRs reporting efficacy, there were improvements in Hb change from baseline, Hb response, and reduction in RBC transfusions for patients with CIA treated with ESAs vs. control. Results were less consistent across SRs for mortality, thromboembolic events, and HRQoL for ESA vs. control. Lack of study quality reporting may affect the strength of these findings.
Morga: Astellas Pharma Europe Ltd.: Current Employment. Atzinger: Astellas Pharma Inc.: Current Employment. Arber: York Health Economics Consortium Ltd: Current Employment. King: York Health Economics Consortium Ltd: Current Employment. Phalguni: York Health Economics Consortium Ltd: Current Employment. Sanderson: York Health Economics Consortium Ltd: Current Employment. Alexandre: Astellas Pharma Europe B.V.: Current Employment.